Answer: 114.29 mL
Step-by-step explanation:
We are given that density of ether = 0.70 g/ml
Mass of ether= 80.0 g
Formula to calculate the density is given by :-
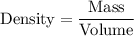
Substitute the value of density and mass , we get
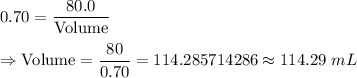 [Rounded to the nearest two decimal places.]
[Rounded to the nearest two decimal places.]
Hence, the volume of ether = 114.29 mL