Answer: Option (a) is the correct answer.
Step-by-step explanation:
When only one element gets displaced upon chemical reaction between a compound and an element then it is known as a single replacement reaction.
For example,
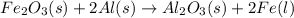
On the other hand, when two compounds chemically react together and their ions get replaced by each other then it is known as a double replacement reaction.
For example,
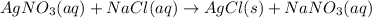
Thus, we can conclude that the reaction which has two reactants with ions that seem to switch places clue can be used to identify a chemical reaction as a replacement reaction.