Answer:
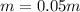
Step-by-step explanation:
Hello,
In this case, molality is defined as:
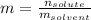
Whereas the mass of the solvent is given in kilograms. In such a way, for 5 moles of solute and 100 kg of water, the molality turns out:
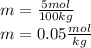
Now it is important to notice that the molal units (m) equals mol/kg, thereby:
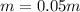
Best regards.