Answer:
119.69 kilo liter volume of methane measured at STP, must be burned.
Step-by-step explanation:
Length of the pool= 10.0 m
Breadth of the pool = 4.0 m
Height upto which the pool will be filled= 3.0 m
Volume of the water in the pool = volume of the pool :
= 10.0 m × 4.0 m × 3.0 m =
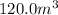
Density water =
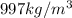
Mass of the water(m) =
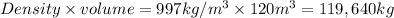
Energy required to raise the temperature = Q
Mass of the water = m = 119,640 kg =
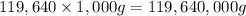
Specific heat of water ,c= 4.186 J/g °C
Change in temperature,
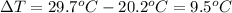
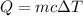
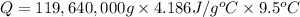
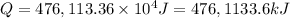
Heat required to raised the temperature is 476,1133.6 kJ.
Heat evolved when 1 mol of methane = -891 Kj
Number of moles of methane giving (- 476,1133.6) kJ of heat:
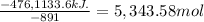
At STP, 1 mol occupies = 22.4 L
Then 5,343.58 mol will occupy :
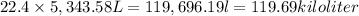
119.69 kilo liter volume of methane measured at STP, must be burned.