Answer: In the given sample, 0.533 moles of hydrogen will be present.
Step-by-step explanation:
We are given a chemical compound having formula
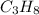
We are provided with 0.20 moles of carbon atoms.
As, for every 3 moles of carbon atoms, 8 moles of hydrogen atoms are present.
So, for 0.20 moles of carbon atoms,
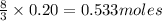 of hydrogen atoms will be present.
of hydrogen atoms will be present.
Hence, in the given sample, 0.533 moles of hydrogen will be present.