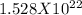Answer:
number of gold atoms =
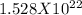
Step-by-step explanation:
The mass of one atom of gold is given to be =
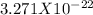
The mass of gold is given to be =
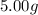
In order to determine the number of atoms present in the 5 g of gold, we will divide the mass of the gold with the mass of each atom of the gold.
the number of atoms =
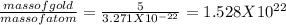
Thus number of gold atoms =