Answer :
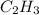 or 3.97
or 3.97
Explanation : As the the molar mass of carbon and hydrogen are ;
C=12 g/mol and H =1 g/mol
So, When Carbon Hydrogen mass ratio is 11.89
Then ratio of no. of atoms of C and H is given by
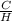 =
=
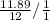
Which will be ≅ 1
So empirical formula C H is now possible
When Carbon Hydrogen mass ratio is taken as 3.97
The ratio of no. Of atoms of C and H is given by
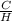 =
=
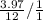
which gives the answer as
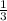
So empirical formula
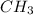 is possible
is possible
It can be cross verified as
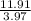 ≅ 2.99.
≅ 2.99.
Therefore 3.97 is the correct answer and the empirical formula will be as
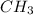 or
or
