Answer: The particle unit is an ion.
Explanation: The unit which is common used for the substances is Mole. 1 mole of anything equals to Avogadro number which is
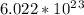 .
.
For example, 1 mole of an electron equals to
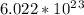 electrons,
electrons,
1 mole of an atom equals to
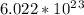 atoms.
atoms.
So, 1 mole equals to
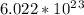 particles.
particles.
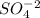 is sulfate ion. So, the particle unit for this will be an ion.
is sulfate ion. So, the particle unit for this will be an ion.