Answer: Option (A) is the correct answer.
Step-by-step explanation:
It is known that acids are the species that donate hydrogen ions (
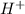 ) or protons upon dissociation in an aqueous solution.
) or protons upon dissociation in an aqueous solution.
For example,
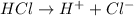
Also, bases are the species which are electron rich and hence they donate electrons to an electron deficient atom.
For example,
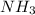 is a base.
is a base.
Therefore, we can conclude that out of the given options correct pairing is acid: proton; base: electron pair.