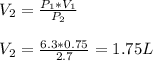Answer:
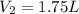
Explanation:
Use the Boyle's law which states that the pressure of a gas in a closed container is inversely proportional to the volume of the container, when the temperature is constant. Mathematically this is:
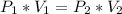 (1)
(1)
Where:
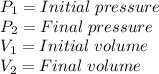
Before attempting to solve the problem, let's do the pertinent conversions:
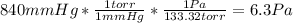
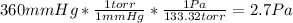
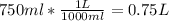
So, we got:
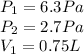
Now, let's isolate
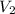 from (1) in order to find the final volume:
from (1) in order to find the final volume: