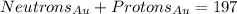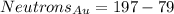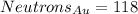Answer:
79 protons, 79 electrons and 118 neutrons
Step-by-step explanation:
Hi, the atomic number of an element is equal to the number of protons it has.
For gold (Au):
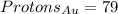
If the atom is uncharged (and only if it is uncharged), the number of electrons it has is equal to the number of protons.
For gold (Au):
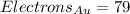
At last, the atomic mass represents the protons plus the neutrons an atom has in its core. So: