Answer:
See explanation.
Step-by-step explanation:
Hello,
In this case, an excited state means is referred to the valence electron that has moved from its lower state orbital, at which the lowest available energy is present, to another orbital with higher energy.
In this manner, any electron configuration in which the last electron is located at an orbital with higher energy, stands for an element at an excited state. For instance, looking at the lower state of nitrogen, the resulting electron configuration turns out:
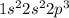
Now, by exciting the element, an electron could occupy a large number of orbitals. Nonetheless, it will occupy the next available one, as shown below:
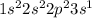
Wherein the valence electron is now at the
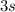 orbital in the so called excited state.
orbital in the so called excited state.
Best regards.