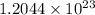Answer : The number of chloride ions present in 0.100 mole of
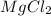 are,
are,
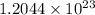
Explanation : Given,
Moles of
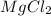 = 0.100 mole
= 0.100 mole
As we know that, 1 mole contains
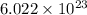 number of ions.
number of ions.
In
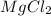 , there are one magnesium ion and two chloride ions.
, there are one magnesium ion and two chloride ions.
As, 1 mole
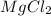 contains
contains
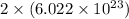 number of chloride ions.
number of chloride ions.
So, 0.100 mole
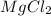 contains
contains
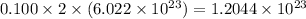 number of chloride ions.
number of chloride ions.
Therefore, the number of chloride ions present in 0.100 mole of
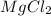 are,
are,