Answer:
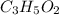 the empirical formula of a compound.
the empirical formula of a compound.
Step-by-step explanation:
Suppose in 100 g of compound contains 50.0% carbon, 6.7% hydrogen, and 43.3% oxygen;
Then mass of carbon =
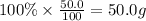
Moles of carbon =
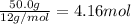
Then mass of hydrogen=
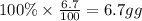
Moles of hydrogen=
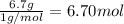
Then mass of oxygen =
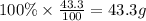
Moles of oxygen =
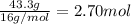
To determine the empirical formula compound dived the smallest value of moles from each moles of element.
Carbon =
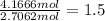
Hydrogen=
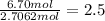
Oxygen=
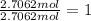
The empirical formula ;
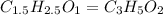
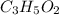 the empirical formula of a compound.
the empirical formula of a compound.