We are given the following information
Specific heat capacity of water = 4184 J/kg.°C
Amount of heat = 31,840 Joules
Change in Temperature = 22.0 °C to 28.5 °C
We are asked to find the mass of the water in grams.
Recall the specific heat capacity formula is given by
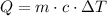
Let us substitute the given values and solve for mass (m)
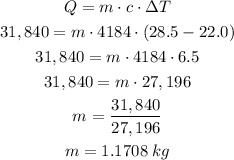
Multiply the mass in kg by 1000 to convert into grams.
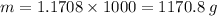
Therefore, the mass of the water is 1170.8 grams.