Answer:
D) V-nb
Step-by-step explanation:
The ideal gas equation is given as:
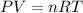
where P = pressure, V = volume, n = number of moles
T = temperature and R = gas constant
The ideal gas equation assumes that the gases behave as point masses and that they are so farther apart that they do not experience any intermolecular forces.
However, real gases have a definite volume and are subject to intermolecular forces of attraction. This is accounted for in the Vander waals equation by introducing two constants:
a = correction for intermolecular forces
b = correction for volume
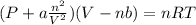
when a = b = 0 the above equation approaches the ideal gas equation.
Thus, the volume correction = V-nb