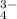Sodium/natrium is a metal from first column group so it should have one 1+ charge. Phosphate ion has 3- charge. That is why there 3 natrium ion for 1 phosphate ion when this molecule is dissolved in water. The ion formula would be:
(Na)

(PO

) ==> 3 Na

+ PO