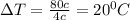Answer: C) The temperature change will be approximately 20°C.
Step-by-step explanation:

Q= heat gained
m= mass of the substance = 2 kg
c = heat capacity = c J/g ° C
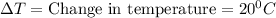
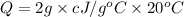
Q=40c Joules
That is in 3 minutes, heat absorbed = 40 c joules
in 6 minutes, heat absorbed=

Now as the substance is same, the heat capacity will be same.