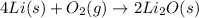Answer: The word equation is given below.
Step-by-step explanation:
Word equation is defined as the chemical equation in which chemical substances are written in words rather than in their chemical formulas.
So, the word equation for the reaction of lithium and oxygen to form lithium oxide is:
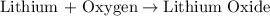
The balanced chemical equation with their chemical formulas is given as: