Answer: Calcium atom has 2 valence electrons.
Step-by-step explanation:
Valence electrons are defined as the electrons which are present in an outermost shell of an atom. It is determined by the electronic configuration of an atom.
Calcium is the 20th element of the periodic table. The electronic configuration of this atom will be:
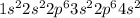
As, outermost shell which is '4s' has 2 electrons.
Thus, calcium atom has 2 valance electrons.