Answer:
The formula of the gas produced is
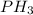 .
.
Step-by-step explanation:
Whenever metal phosphides reacts with water they form phosphine gas and alkaline solution of metal hydroxide.
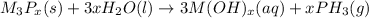
So, when sodium phosphide is treated with water it gives phosphine gas and sodium hydroxide solution as a product.
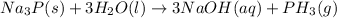
The formula of the gas produced is
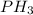 .
.