Answer: The correct answer is
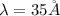 .
.
Step-by-step explanation:
The expression for the relation between the energy of the wave and the wavelength is as follows;
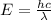
Here, E is the energy of the wave, h is Planck's constant, c is the speed of the light and
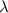 is the wavelength of the wave.
is the wavelength of the wave.
It is given in the problem that the energy of the photon is
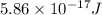 .
.
Put c=
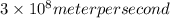 ,
,
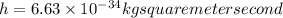 and
and
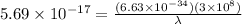
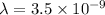
Convert the value of the wavelength into angstrom.
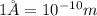
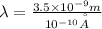
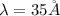
Therefore, the wavelength of a photon is
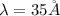 .
.