Answer: Correct options are as follows.
- salt is not chemically bonded to water.
- salt and water retain their own chemical properties.
Step-by-step explanation:
When salt is dissolved in water then it means that it is a physical change as salt has completely dissociated into ions but they are not chemically combined to the water molecules.
As a result, both salt and water will retain their chemical properties.
For example, NaCl when dissolved in water will dissociate as follows.
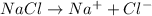
Only the particles of salt have evenly distributed in water.
And, when a components of a salt chemically combine with another substance then it will form a new compound.
Therefore, we can conclude that salt dissolved in water is a solution, therefore:
- salt is not chemically bonded to water.
- salt and water retain their own chemical properties.