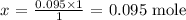Answer:
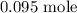
Step-by-step explanation:
Here, we want to know the number of moles of hydrogen in the given mass of sucrose
To get this, we need to get the actual mass of the total that is hydrogen
Mathematically, we have that as:

Thus, we have the mass of hydrogen in the sucrose as:
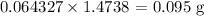
1 mole of hydrogen has a mass of 1g
x mole will have a mass of 0.095g
Thus,