Answer: 65.9 %
Step-by-step explanation:
The formula for calculating percentage yield is:
Percentage yield =
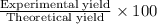
Given : Experimental yield = 14.5 g
Theoretical yield = 22.0 g
Putting in the values we get:
Percentage yield =
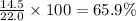
Therefore, the percentage yield is 65.9 %.