Molar mass of C= 12g/mol
2C= 2*12g/mol= 24g/mol
Molar Mass of F=19g/mol
4F= 4*19g/mol= 76g/mol
Total molar mass of C2F4 = (24g/mol+76g/mol)= 100g/mol
Now you need to find the moles of Fluorine
Moles= Mass/molar mass
Moles of Fluorine = 5.71g/100g/mol=0.0571mol of Fluorine
To find the number of atoms you multiply moles by Avogadro's number(
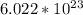
)
So the number of Fluorine atoms= 0.0571* 6.022x10^23= 3.44x10^22 atoms or
 atoms
atoms