Answer:
5e6 atoms
Step-by-step explanation:
Hello,
At first, we must convert from pm to mm to find the diameter of the chlorine atom in mm:
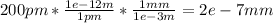
Now, by dividing the total distance by the distance of one chlorine atom, we can compute how many chlorine atoms are required as they are aligned:
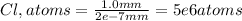
Best regards.