Answer:
Bromate anion is obtained buy removing one oxygen atom from the perbromate ion
Step-by-step explanation:
The given ion of per bromate ion =
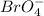
In the perbromate ion the oxidation state of bromine atom is of +7.
On removing an oxygen atom from the given polyatomic anion we will have:
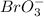 ion that is bromate ions.
ion that is bromate ions.
In the bromate ion the oxidation state of bromine atom is of +5.
The structure of both anions are shown in an image attached.