Answer
NaOH is the limiting reactant
Step-by-step explanation
Given reaction:
2 Cl₂ + 4 NaOH → 3 NaCl + 1 NaClO₂ + 2 H₂O
2 mol of Cl₂ = 71.0 g
4 mol of NaOH = 160.0 g
What to find:
The limiting reactant.
Step-by-step solution:
From the given reaction; 71.0 g of Cl₂ reacted with 160.0 g of NaOH
So the given reacting mass (11.9 g) Cl₂ is expected to react with:
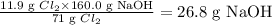
Note that the given mass of NaOH is 12.0 g but the actual mass needed to consume 11.9 g Cl₂ is 26.8 g. Hence, Cl₂ is the excess reactant and NaOH is the limiting reactant