Answer : The mass of potassium chlorate is, 102.125 grams
Step-by-step explanation:
Potassium chlorate is a compound that contains potassium, chlorine and oxygen atom. The molecular formula potassium chlorate is,
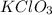
From the molecular formula potassium chlorate we conclude that, there are 1 atom of potassium, 1 atom of chlorine and 3 atoms of oxygen.
The mass of 3 oxygen atom = 3 × 16 = 48 g
The molar mass of potassium chlorate = 122.55 g/mole
As, 48 gram of oxygen present in 122.55 grams of potassium chlorate
So, 40 gram of oxygen present in
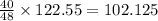 grams of potassium chlorate
grams of potassium chlorate
Therefore, the mass of potassium chlorate is, 102.125 grams