Answer: The mass of oxygen atom that will react with given amount of benzene will be 32.17 grams.
Step-by-step explanation:
To calculate the number of moles, we use the equation:
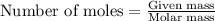 .....(1)
.....(1)
For Benzene:
Given mass of benzene = 10.47 g
Molar mass of benzene = 78.1074 g/mol
Putting values in above equation, we get:
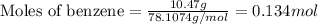
For the given chemical reaction:
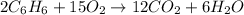
By stoichiometry of the reaction:
2 moles of benzene reacts with 15 moles of oxygen.
So, 0.134 moles of benzene will react with
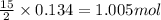 of oxygen.
of oxygen.
Now, to calculate the mass of oxygen, we use equation 1
Moles of oxygen gas = 1.005 mol
Molar mass of oxygen gas = 32 g/mol
Putting values in equation 1, we get:
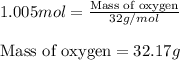
Hence, the mass of oxygen atom that will react with given amount of benzene will be 32.17 grams.