Answer:
An electron has charge but negligible mass, whereas a neutron has mass but no charge.
Step-by-step explanation:
Atoms are made of equal number of protons and electrons. Almost all the mass of an atom is in the nucleus of the atom. The nucleus is made of protons and neutrons (except hydrogen, which has no neutron)
The charge of an electron is
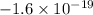 C
C
The charge of a proton is
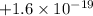 C
C
The charge of a neutron is 0 C
The mass of a proton is
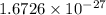 kg or 1.007276 u
kg or 1.007276 u
The mass of a neutron is
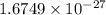 kg or 1.008664 u
kg or 1.008664 u
The mass of an electron is
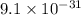 kg or 0.00054858 u
kg or 0.00054858 u
As we can see the proton is 1,838 times heavier than an electron and the neutron is 1,840 times heavier than an electron.
So the electron has a negligible mass and a negative charge but the neutron has no charge but it is heavier than electron and proton