Answer:
0.144 L (144 mL).
Step-by-step explanation:
First, let's calculate the molarity of the solution of 0.600 L of water with 50.0 g of NaOH. But first, let's calculate the number of moles of 50.0 g of NaOH using its molar mass which is 40 g/mol (you can calculate the molar mass of a compound using the periodic table):
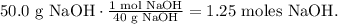
The next step is to use the formula of molarity:
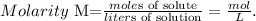
And replace the given data (moles of solute = 1.25, liters of solution = 0.600 L):
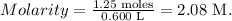
We want to find the volume that is needed to create a 1.50 L (volume) of 0.200 M NaOH. We have to use the formula:

Where C indicates the concentration in M and V the volume in L. In this case, our unknown value can be V1 but remember that the concentration of this solution (C1) was 2.08 M and we have to equal this to the concentration (0.200 M) and volume (1.50 L) of the wanted solution. It will look like this:
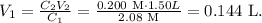
The volume required would be 0.144 L (144 mL).