Density is defined as the ratio of mass to the amount of matter i.e. volume.
The mathematical expression is given as:
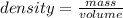
Now, from the mathematical expression, density is inversely proportional to volume and directly proportional to mass implies with decrease in volume, density is also decreases and with increase in mass, density is also increases.
Low volume (compression) is due to high pressure, results in increase in density and high temperature results large volume (expansion) implies temperature is inversely proportional to density.
Thus, pressure is directly related to density implies increase in pressure results in increase in density.
Hence, first option is the correct answer.