Answer: Hydrogen can only form 1 atom.
Step-by-step explanation:
Hydrogen is the 1st element of the periodic table having electronic configuration of
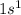
This element cannot loose 1 electron because after that, this element will be left with 0 electrons and that will lead to instability of an atom. So, it will only form covalent bond.
A covalent bond is formed when sharing of electrons takes place between the atoms forming the bond. For Example: HCl
As, hydrogen require only 1 electron to attain stable electronic configuration. Thus, it will only form 1 bond (having 2 electrons) with any other atom.