Answer : The atomic mass in
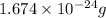 of neutrons are, 1.008 amu.
of neutrons are, 1.008 amu.
Explanation :
1 mole of substance always contains
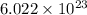 number of atoms. The mass of one moles of any substance is equal to the atomic mass unit.
number of atoms. The mass of one moles of any substance is equal to the atomic mass unit.
As we know that,
1 gram =
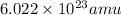
As we are given that:
Mass of neutrons =
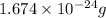
Now we have to determine the number of atomic mass unit.
As, 1 gram =
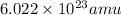
So,
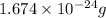 =
=
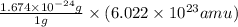
= 1.008 amu
Therefore, the atomic mass in
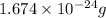 of neutrons are, 1.008 amu.
of neutrons are, 1.008 amu.