Step-by-step explanation:
Loss of one or more electrons by a neutral atom will help the neutral atom to acquire a positive charge.
For example, atomic number of sodium is 11 and to stabilize itself it needs to lose one electron. So, by losing an electron it will change into
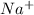 ion.
ion.
On the other hand, gain of one or more electrons by a neutral atom will help the neutral atom to acquire a negative charge.
For example, Cl has atomic number 17 and to fill its octet completely it need one more electron. So, by gaining an electron is will change into
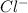 ion.
ion.
Therefore, we can conclude that an ion is an atom with a net electrical charge due to loss or gain of electrons.