Answer: 1.1 atm
Step-by-step explanation:
Using ideal gas equation:
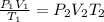
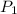 = initial pressure = 0.98 atm
= initial pressure = 0.98 atm
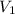 = initial volume =
= initial volume =
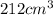
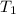 = initial temperature= 293 K
= initial temperature= 293 K
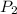 = final pressure = ?
= final pressure = ?
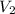 = final volume=
= final volume=
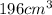
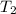 = final temperature=308 K
= final temperature=308 K
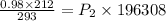
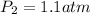
Thus final pressure of the gas is 1.1 atm.