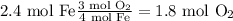Answer
1.8 mol O₂
Procedure
Considering the following chemical reaction:
4Fe + 3O₂ → 2Fe₂O₃
To solve the exercise we will assume that oxygen is in excess and iron is the limiting reaction.
Based on this and the stoichiometry of the reaction we have the following equation: