Answer: The correct answer is substitution reaction.
Step-by-step explanation:
Addition reaction is the chemical reaction where atoms get added to the given compound. No atom is lost during this particular reaction. In organic chemistry, only unsaturated hydrocarbons (alkenes and alkynes) shows this type of reaction.
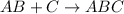
Combustion reaction is a chemical reaction where a hydrocarbon reacts with oxygen gas to produce carbon dioxide gas and water molecule.
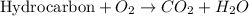
Substitution reaction is a chemical reaction where one atom replaces the other atom from the given compound. In organic chemistry, only saturated hydrocarbons (alkanes) favors this reaction.
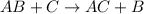
Hydration reaction is a chemical reaction in which hydrogen atoms get added to the compound. This reaction is favored by unsaturated hydrocarbons only (compounds which have multiple bonds).

So, the reaction which is important only for alkanes is substitution reaction.