Answer: B. CO
Step-by-step explanation:
Diatomic molecules are those that are formed by two atoms of the same chemical element (homonuclear diatomic molecule) or different chemical element (heteronuclear diatomic molecule).
In this sense, oxygen is a homonuclear diatomic molecule because it is formed by two atoms of the same element (
 ) and Carbon monoxide (
) and Carbon monoxide (
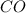 ) is heteronuclear diatomic molecule.
) is heteronuclear diatomic molecule.
Sodium Chloride
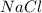 is not a diatomic molecule because is a product of ionization, but it can be diatomic in its gas phase with a polar covalent bond.
is not a diatomic molecule because is a product of ionization, but it can be diatomic in its gas phase with a polar covalent bond.