Answer: Option (B) is the correct answer.
Step-by-step explanation:
Atomic number of lithium is 3 and its electronic distribution is 2, 1.
Atomic number of bromine is 35 and its electronic distribution is 2, 8, 18, 7.
It is known that a neutral atom which accepts electrons acquires a negative charge and a neutral atom that loses electrons will acquire a positive charge.
So, in order to attain stability lithium will lose its 1 valence electron and bromine will accept its 1 electron.
Therefore, lithium will acquire a positive charge and bromine will acquire a negative charge.
Thus, we can conclude that electrical charges would the 2 newly formed ions take on is
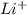 and
and
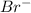 .
.