Answer: The element having given electronic configuration is palladium.
Step-by-step explanation:
Electronic configuration is defined as the representation of electrons around the nucleus of an atom. This representation also helps in the determination of atomic number of an element.
We are given:
Electronic configuration of unknown metal:
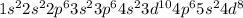
Total number of electrons present in the atom = [2 + 2 + 6 + 2 + 6 + 2 + 10 + 6 + 2 + 8] = 46
The element having atomic number 46 is palladium.
Hence, the element having given electronic configuration is palladium.