Answer: The density of metal is 8.97 g/mL
Step-by-step explanation:
Density of a substance is defined as the ratio of its mass and volume. The chemical equation representing density of a substance is:
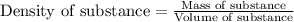 .......(1)
.......(1)
Mass of sample 1 = 35.71 g
Volume of sample 1 = 3.9 mL
Putting values in equation 1, we get:
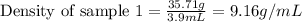
Density of sample 1 = 9.16 g/mL
Mass of sample 2 = 39.04 g
Volume of sample 2 = 4.5 mL
Putting values in equation 1, we get:
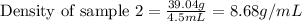
Density of sample 2 = 8.68 g/mL
Mass of sample 3 = 40.81 g
Volume of sample 3 = 4.5 mL
Putting values in equation 1, we get:
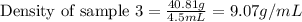
Density of sample 3 = 9.07 g/mL
- To calculate the density of metal, we take the average of the densities:
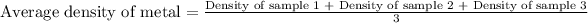
Putting values in above equation, we get:
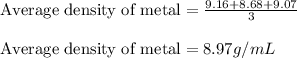
Hence, the density of metal is 8.97 g/mL