Answer : The products formed on the decomposition of ammonia are, hydrogen gas
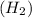 and nitrogen gas
and nitrogen gas
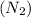 .
.
Explanation :
Decomposition reaction : It is defined as the chemical reaction in which the larger molecules decomposes to give two or more smaller molecules.
When the ammonia
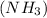 decomposes then it gives hydrogen gas and nitrogen gas as a product.
decomposes then it gives hydrogen gas and nitrogen gas as a product.
The balanced chemical reaction will be,
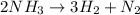
In this reaction,
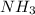 is the reactant and
is the reactant and
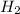 and
and
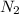 are the products.
are the products.
By the stoichiometry we can say that 2 moles of ammonia decomposes to give 3 moles of hydrogen gas and 1 mole of nitrogen gas as a product.
Therefore, the products formed on the decomposition of ammonia are, hydrogen gas
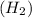 and nitrogen gas
and nitrogen gas
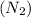 .
.