Answer : The correct option is, Electrons are transferred.
Explanation :
Iron rusting : It is a chemical process in which the an iron nail react with water and oxygen to give iron oxide as a product. Rusting of iron is an oxidation-reduction reaction in which iron losses electrons to oxygen atom.
Oxidation-reduction reaction : It is a reaction in which oxidation and reduction reaction occur simultaneously.
- Oxidation reaction : It is the reaction in which a substance looses its electrons. In this oxidation state increases.
- Reduction reaction : It is the reaction in which a substance gains electrons. In this oxidation state decreases.
The balanced chemical reaction for rusting of irons is,
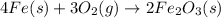
Half reactions of oxidation and reduction are :
Oxidation :
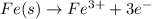
Reduction :
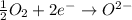
From this reaction we conclude that the electrons are getting transferred from iron to oxygen.
Hence, the correct option is, Electrons are transferred.