Answer: The given reaction is an example of exothermic reaction.
Step-by-step explanation:
There are two types of thermochemical reactions:
1. Endothermic reactions: These are the type of reactions in which energy of the products is higher than the energy of the reactants. Energy is absorbed during this reaction and the energy term is written on the reactant side. Total enthalpy change of the reaction comes out to be positive.
2. Exothermic reactions: These are the type of reactions in which energy of the products is lower than the energy of the reactants. Energy is released during this reaction and the energy term is written on the product side. Total enthalpy change of the reaction comes out to be negative.
For the given chemical reaction:
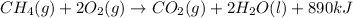
The energy term is written on the product side. Thus, energy is being released during this reaction and the reaction is considered as an exothermic reaction.