Step-by-step explanation:
It is known that isotopes are the species which have same atomic number but different mass number. Whereas a specie which have different atomic number but same mass number are known as isobars.
For example, an element with mass number 35 is chlorine. And, atomic number of a chlorine atom is 17.
This means in a
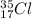 atom there are 17 protons and the number of neutrons will be calculated as follows.
atom there are 17 protons and the number of neutrons will be calculated as follows.
Mass number = no. of protons + no. of neutrons
35 = 17 + no. of neutrons
No. of neutrons = 35 - 17
= 18
Hence, we can conclude that the isotope symbol with mass numbers of 35 is
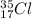 .
.