Answer:
The number 4 indicates the mass number of the element.
Step-by-step explanation:
The mass is defined as the number of nucleons in the nucleus of the element.
Let an element is
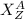
Here Z is the number of protons in the nucleus
A be the number of nucleons in the nucleus
The number of nucleons is the sum of number of protons and the number of neutrons.
So, the number of protons in the nucleus are Z, number of neutrons are A - Z in the nucleus.
Thus, 4 indicates the sum of number of protons and the neutrons.