Step-by-step explanation:
According to the ideal gas law, PV = nRT
where, n = number of moles
Also, density =
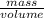
Since, n =
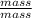
Therefore, ideal gas equation can also be written as follows.
PV = nRT
PV =
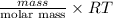
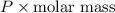 = dRT
= dRT
Hence, putting the given values into the above formula as follows.
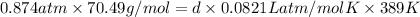
6.161 atm g/mol =
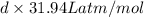
d = 0.193 g/L
Thus, we can conclude that density of the given gas is 0.193 g/L.